When healthcare workers and residents and staff at long-term care facilities received the first COVID-19 vaccinations in mid-December 2020, the world marveled at the speed with which both the Pfizer-BioNTech and Moderna mRNA-1273 vaccines were developed. Less heralded, though equally significant, were the behind-the-scenes processes to ramp up drug production and distribution. Valve suppliers played a key role in helping to ensure timely, safe manufacturing of SARS-CoV-2 vaccinations.
One of the companies that has seen a spike in business related to COVID-19 is ITT Engineered Valves. The majority of ITT’s customers are in the biopharmaceutical market, producing medical drugs derived from proteins and nucleic acids for therapeutic or in vivo diagnostic purposes. The company felt well-prepared to step up during the coronavirus pandemic, particularly given its experience in the vaccine production process.
When the H1N1 virus emerged in 2009, ITT worked with a customer contracted by the U.S. government to build a new vaccine facility. “That generated about $2 million worth of valves in a very short time period,” said Dave Loula, global product director of ITT. Fast forward 11 years and the company once again landed business during a pandemic. However, it looked a bit different.
“We expected to see significant capacity needs as a result of COVID-19. But in terms of drug production, we haven’t seen a big groundswell of demand,” said Loula. “The real demand is on the filling and finishing side.” As the name suggests, filling and finishing—or fill-finish—is the process of filling vials with vaccine and packaging the medicine for distribution. ITT has several projects with companies subcontracted to perform fill-finish.
“These projects have had to move at the same accelerated rate as vaccines going through trials and approvals because as soon as the drugs are approved, companies want to get them filled, packaged and moved to distribution points to get the vaccinations out to people,” said Loula.
A Speedy Production Cycle
Leading the drive for fast development, production and distribution of vaccinations is Operation Warp Speed, a partnership among several entities of the Department of Health and Human Services working hand-in-hand with other federal agencies and private firms. The goal of the partnership is to make 300 million doses of COVID-19 vaccine available by January 2021.
“One of the challenges throughout the biopharmaceutical market is the pace and speed required for general project execution,” said Matt Sullen, regional manager at PBM Inc. Valve Solutions. His company sells a variety of valves used in both upstream and downstream processes to several companies in the biopharmaceutical market. “The customer base needs to get their products to market quickly, and COVID vaccines are just one example. You could see the effects all the way from diagnostics to manufacturing to fill, finish and distribution. As a valve supplier, you need to deliver.”
In April 2020, Richards Industrials rolled out an expedited manufacturing process, as did other valve manufacturers, to help biopharmaceutical customers streamline production. “If a customer is expanding a plant to make vaccines as part of BARDA [the Biomedical Advanced Research and Development Authority] or Operation Warp Speed and they need valves quickly, we’ll do what it takes to make that delivery happen as fast as it can,” said Karl Lutkewitte, product and sales manager at Steriflow Valve Division of Richards Industrials. To meet demand, the company has often had to deliver 50 valves in two weeks or less.
Global supply chain disruptions common during the COVID-19 pandemic sometimes thwarted efforts. Emerson Automation Solutions experienced a significant increase in business in the overall medical market in 2020, initially in the medical device segment as demand for products such as ventilators skyrocketed, then in biomanufacturing as vaccine production took off. “Emerson has had to not only shift production around to meet these demands, but also manage a complex supply chain to ensure production could continue to meet those demands,” said Don Launder, director of global strategic accounts—hybrid.
Design Challenges and Service Conditions
Even amid the global pandemic, valve and control product suppliers continued to meet demand in the burgeoning biopharmaceutical market unrelated to COVID-19. One of ITT’s biggest projects in 2020 was for a company increasing its diabetes drug production. ITT collaborated with the end user, equipment OEM and contractors to help specify and design valves using new technology developed to streamline the installation and maintenance process. ITT delivered more than 1,500 valves.
No matter what a biopharmaceutical company is producing, there are a few noteworthy challenges that valve suppliers face related to the regulatory landscape, design requirements and the production environment. As in most market segments, adhering to regulations is paramount.
“In biopharma manufacturing, especially on process valves, there are a number of regulations that must be met due to the fact that the valve can be in contact with drug substances or products,” said Launder. The primary regulatory body is the Food and Drug Administration (FDA), and the American Society of Mechanical Engineers (ASME) publishes the ASME-BPE standard detailing requirements for bioprocessing equipment.
“Any piping and valves used by [a biopharmaceutical company] have to be certified,” said Jeff Kane, director of sales and marketing at DFT Inc., which supplies non-slam check valves to the sanitary industry. “Once the system has been certified, companies don’t have a whole lot of options to make a change. They have to make the right choice from the beginning.”
Making the right choice can be challenging as there are a lot of design decisions. “From diaphragm life and ease of change-out to on-board diagnostics and overall valve size and complexity, there are nearly endless possibilities,” said Launder. “Customer requirements and usage is key to determining these design features.”
Maintaining a sterile environment is a primary concern for biopharma manufacturers since they create products from live cells or their components. All the stainless-steel processing systems must be thoroughly cleaned before the next batch of drugs is manufactured. They are typically sanitized in a clean-in-place (CIP) process, then sterilized in a steam-in-place (SIP) process, with valves controlling both processes. “The primary service concern is a sterile environment, where nothing gets entrapped in the system,” said Lutkewitte.
Market Trends
There are several trends in the biopharmaceutical industry affecting valve and control product suppliers. One is the move toward single-use components. Instead of growing cells in a stainless-steel bioreactor, companies will use a large disposable bioreactor made of layered plastic materials. The bags range from approximately 500 to 4,000 liters, with one manufacturer recently introducing a 6,000-liter bag, according to Lutkewitte.
“Single-use production practices reduce the overall cost and complexity of drug manufacturing, primarily through eliminating cleaning and the impacts of cleaning and sterilizing,” said Launder. While the shift away from stainless-steel bioreactors reduces the need for process control valves, disposable bioreactors use a lot of tubing that requires pinch valves.
Biopharmaceutical companies also are striving to downsize their systems and facilities. “Customers are always looking to shrink the envelope of valve assembly. They try to tighten up their piping systems to reduce dead leg and waste,” said Loula. “So we try to continue to develop products that meet those smaller and smaller requirements.”
In addition to paying attention to product-specific trends, valve suppliers keep a close eye on global trends in the biopharmaceutical industry that affect their business. One is the dramatic rise of cell and gene therapies. Between 2018 and 2024, the compound annual growth rate of cell and gene therapies in North America is forecast to be 28.4%, according to a May 2020 article in Genetic Engineering & Biotechnology News. “With cell and gene therapy taking off, the number of gas regulator valves we sell has gone through the roof,” said Lutkewitte.
Personalized medicine, also known as precision medicine, is another emerging practice. Physicians use a patient’s genetic profile to choose the proper medication or therapy. “It’s still early in the game for personalized medicine, but it’s coming,” said Lutkewitte.
One biopharma segment that’s already flourishing is vaccines. While COVID-19 vaccines are currently grabbing the headlines, others have experienced increased dissemination in recent years. These include annual influenza shots, shingles vaccinations and the human papillomavirus (HPV) vaccine to protect against certain cancers. “Vaccines and other medical therapies are driving biotechnology on a global basis,” said Loula.
A Bullish Outlook
The future looks bright for the biopharmaceutical industry and, by extension, the valve manufacturers that serve the industry. “We are bullish on the biopharmaceutical market for the next three years,” said Lutkewitte. “It’s a growing industry as long as researchers develop new drugs.”
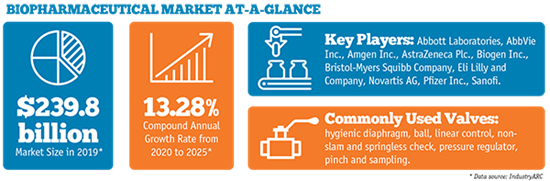
The data supports that research and development will continue at a robust pace. The biopharmaceutical industry employed more than 290,000 people in R&D in 2017—the largest number of R&D workers in any U.S. industry, according to a report published by the Pharmaceutical Research and Manufacturers of America (PhRMA) and TEConomy Partners. That figure accounts for 36.2% of all biopharma industry jobs and is nearly three times higher than the U.S. industry average R&D employment, according to PhRMA.
“As more drugs in the biopharma sector are consumed, more of the equipment used to produce them is going to be needed,” said Loula. That’s good news for valve suppliers in the biopharmaceutical market.
RELATED CONTENT
-
Misconceptions Regarding Control and Isolation Valve Standards
All on/off isolation valve standards are not created equal and cannot be applied unilaterally to all valves.
-
Intermediate Class Valves, the Forgotten Classification
These days, piping designers use automated systems that default to standard classifications such as pressure classes of 150 to 2500 for valves and associated equipment.
-
What’s Your Temperature?
For decades, valve manufacturers have provided the maximum recommended working pressures and temperatures for their products, based on the materials used in the pressure-containing parts.










 Unloading large gate valve.jpg;maxWidth=214)