Ceramic Coatings Provide Corrosion and Wear Protection for Valves
Ceramic coatings extend the in-service life of equipment by enhancing the corrosion and wear resistance of the base material.
#maintenance-repair #automation
Ceramic coatings such as chrome oxide (Cr2O3) and titanium dioxide (TiO2) applied by thermal spray are frequently used in energy-efficient, highly-corrosive chemical processes including mining and ore extraction. Driven by the industry requirement for low total cost of ownership, lead engineering firms have been and continue to develop innovative solutions such as 1) a nanostructured TiO2 coating, and 2) a ceramic blend of TiO2 and Cr2O3. Most recent results indicate that an optimized balance between the hard and brittle Cr2O3 phases and the soft and ductile n-TiO2 phases results in higher abrasion, sliding wear and galling resistance.
BACKGROUND
Mining is synonymous with erosion and abrasion. In mining, valves are instrumental for transporting slurry, which is a mud composed of solids mixed with water. As opposed to oil and gas flow processes, the solids in mining slurry flow are the desirable materials, whereas the liquid serves as the carrier. In mining, the ratio of solids to liquid can range from 10-80%, compared to around 0.5% to 2% in oil and gas extraction. In a solids-carrying pipeline, the transported material must be ground so it can be suspended in water to achieve a liquid-like flow pattern, with a particle size distribution that can span three orders of magnitude. To reduce friction and mitigate erosion, the flow velocity is typically maintained above the flow velocity at which solids deposition occurs.
After reaching the mineral processing plants, the desired mineral is separated from other elements and impurities that make up the rock. In some cases, this can be accomplished by traditional methods such as smelting; however, energy cost and environment policies have boosted the use of hydrometallurgy—which uses aqueous chemistry. It is within the hydrometallurgy process that we find one of the most demanding valve applications. Here, valves isolate not only pumps but, more importantly, the autoclaves where the prepared and heated slurry begins the pressure-induced chemical reaction that turns rock into precious metals.
These are usually considered severe-service conditions, and they require tailored materials and valve designs for the applications. Whereas many valve types can be optimized for severe-service, MSBVs are usually the preferred choice. MSBV sealing surfaces are inherently protected from particles present in the flow in both open and closed positions, ensuring a reliable shut-off. They are relatively easy to operate in a slurry application because the ball rotates on itself without displacing solids present in the line while tolerating the use of very powerful drive systems. This facilitates rapid closure in critical situations.
The typical MSBV design for hydrometallurgy applications consists of a floating ball in contact with a fixed seat. The constant contact between ball and seats reduces the exposure of the sealing surfaces to the corrosive product. The ball and seats are made of either titanium or duplex stainless-steel substrates protected by a ceramic coating. The primary function of that coating is to enhance the load-carrying capacity and tribological performance of the base material—how interacting surfaces will react to friction and wear. The coating extends the in-service life of the equipment, especially during ball motion phases.
WHY AND HOW R&D HAS OCCURRED
At the start of hydrometallurgy use 20 years ago, conventional Cr2O3 applied by air plasma spraying was the preferred coating for protecting MSBVs from the extreme abrasion, pressure and elevated temperatures inherent to the pressure oxidation (POx) recovery process used in gold processing (gold ore is mixed with oxygen and sulfuric acid in an autoclave). Over time and based on field experiments, silicon dioxide (SiO2) and titanium dioxide (TiO2) were added to the original Cr2O3 blend to improve the ductility and toughness of the coating.
Later still, high-pressure acid leaching (HPAL) was developed and used in the nickel recovery process. The process is similar to POx: Laterite ore is leached in a sulfuric acid environment in an autoclave at 4.1 bar (600 psi) and temperatures above 464ºF (240ºC). However, unlike POx, HPAL operates at high pressures, which, when combined with a higher chloride content, produces a more corrosive environment. (For example, titanium is susceptible to crevice corrosion in HPAL.) The Cr2O3 blend optimized for POx has been found to corrode prematurely when used in HPAL. Because it is relatively inert in this environment, it would seem that TiO2 was a promising choice for HPAL. However, the mechanical and tribological performances of conventional TiO2 are significantly lower than those of Cr2O3, resulting in higher wear rates.
Industry has been working to optimize coating solutions for HPAL. Several options for mitigating corrosion have been considered and tested by various companies, with generally unsuccessful results. Attempts have included the use of intermediate bond coats, such as gold and tantalum, placed between the base material and the Cr2O3 top coat. One engineering firm used a nanostructured TiO2 (nTiO2) coating, which has worked reasonably well. Another firm developed a ceramic blend of TiO2 and Cr2O3 to obtain the corrosion resistance of pure TiO2 and the tribological performance of Cr2O3.
INNOVATIVE TECHNOLOGY
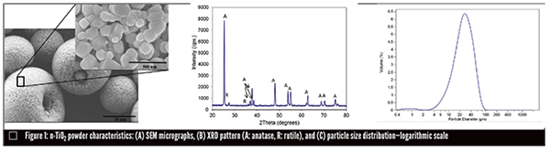
For the NRC/Polytechnique Montréal study, coating materials were selected based on field experience. The novel use of nTiO2 instead of its conventional counterpart was chosen based on recent and promising wear results for this material. The four tested materials were 1) Cr2O3 , 2) TiO2-Cr2O3 , 3) n-TiO2 and 4) n-TiO2-Cr2O3 . All coatings were applied onto titanium grade 5 coupons. The individual n-TiO2 particles used in the powder manufacture (n-TiO2 Millidyne) are smaller than 200 nm (Figure 1), which although finer than conventional thermal spray powders, are within the range of other nanostructured-based powders available for thermal spray processing.
NRC specifically prepared a novel blend and spraying parameters for n-TiO2 and Cr2O3 for this project. The n-TiO2 powder was mixed with a fused, sintered and crushed Cr2O3 powder. The deposition challenge was to ensure that the Cr2O3 particles melt in order to avoid particle rebound when impacting the substrate, as well as erosion of the deposited TiO2 layer by the same particles.
The objective of the project was to assess the mechanical and tribological resistance of these four promising ceramic coatings for hydrometallurgy applications, including the novel n-TiO2-Cr2O3 blend. Hardness and shear strength were determined using micro-hardness indentation testers and universal tensile testing equipment. Wear resistance of the coatings under sliding wear, abrasion and galling conditions were measured by standard pin-on-disc tests, abrasion tests and custom-designed galling tests.
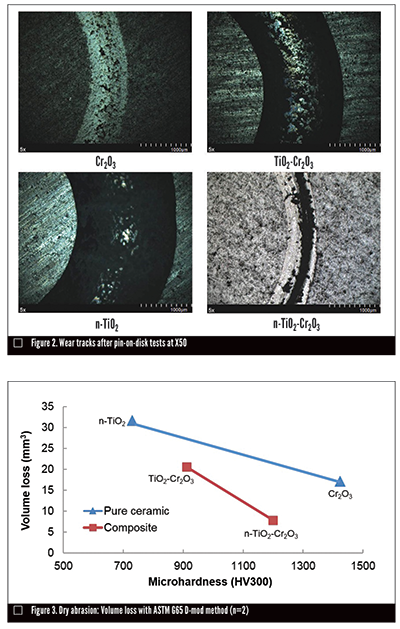
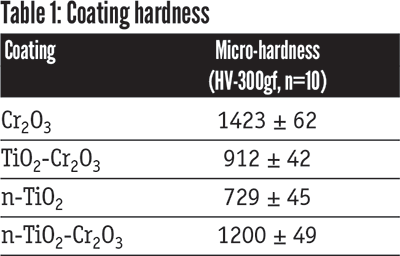
The Cr2O3 ceramic material alone showed the highest hardness and best sliding wear resistance and coefficient of friction. As expected, the Cr2O3 -TiO2 and n-TiO2-Cr2O3 blends produced hardness and sliding wear resistance between what each of the ceramic constituents showed.
These results fall into line with the microscopy observation of the wear tracks after pin-on-disc tests, as shown in Figure 2: the TiO2-Cr2O3 and n-TiO2 coatings have wide and deep wear tracks. In contrast, the Cr2O3 and n-TiO2-Cr2O3 coatings show small wear scars on the shallow surface, indicating mild abrasive wear.
A strong correlation between hardness, friction coefficients and sliding wear resistance was observed; and such a relationship has been frequently documented in the literature.
Previously, tests have shown that improved coating wear performance with added n-TiO2 comes from improved coating toughness. N-TiO2 coatings contain so-called “nanozones”, i.e., regions of unmelted agglomerated n-TiO2 that act as crack arresters.
With respect to galling, the n-TiO2-Cr2O3 coating ranked first, the Cr2O3 , TiO2-Cr2O3 , and n-TiO2 ranked second, third and fourth, respectively.
Overall results indicate that n-TiO2-Cr2O3 provides the best overall tribological performance compared to the other tested ceramics, namely Cr2O3 and n-TiO2. The novel mix of n-TiO2 and Cr2O3 provided consistently superior tribological performance to a TiO2-Cr2O3 blend.
CONCLUSION
For highly corrosive environments that are typically encountered in hydrometallurgy processes, the use of thermal spray ceramic coating such as TiO2 and Cr2O3 applied on corrosion resistant substrates such as titanium or duplex is an established solution.
Leaders are optimizing this existing technology by tuning chemical composition and microstructure to maximize strength, toughness, and corrosion resistance. The novel mix of n-TiO2 and Cr2O3 provides consistently superior tribological performance to a simpler TiO2-Cr2O3 blend. Consequently, this novel blend appears to be a promising evolution. Further optimization and field tests are needed for full commercial deployment.
These results reveal a significant opportunity for increasing customers’ plant uptime and lowering the total cost of ownership by implementing the latest coating methodology. Nevertheless, as each application is unique, customers facing new corrosion challenges would benefit greatly by partnering with a valve manufacturer to strategically develop engineered coating solutions for their specific applications. Although such optimization will typically require testing and qualification, it pays off by increasing customers’ plant uptime, minimizing total cost of ownership, and improving reliability and safety. VM
The authors wish to thank Domonique Poirier and Rogerio Lima (NRC), Jolanta E. Klemberg-Sapieha and Duanji Li (Polytechnique Montréal), as well as David Lee (Kennametal Stellite) for their useful inputs.
Luc Vernhes is director, Innovation, Research and Development and Technology for Velan (www.velan.com). You can reach him at lvernhes@velan.com. Craig Bekins is product manager, Triple-Offset Valves and Nicolas Lourdel is manager, Product Development Process for Velan.
Editor’s Note: A detailed characterization project was undertaken by Velan, in collaboration with the National Research Council of Canada (NRC), Boucherville, and Polytechnique Montréal to assess the mechanical and tribological resistance of promising ceramic coatings. This article reports what the team discovered and why the study was done.
Fundamental Forms of Corrosion
There are many common varieties of corrosion, which show up in a broad array of ways and forms and mostly overlap each other. The mechanism of corrosion resistance is attributed to the formation of a thick protective corrosion film on the metal surface.
The types include:
GALVANIC CORROSION
When two dissimilar metals are in contact and are exposed to a corrosive liquid or electrolyte, a galvanic cell is formed, and the flow of current causes increased corrosion of the anodic member. The corrosion is usually localized near the point of contact. One method to minimize corrosion can be achieved by plating the dissimilar metals.
GALVANIC SERIES:
Anodic, corroded ends
- Magnesium and Magnesium Alloys
- Zinc
- Aluminum
- Mild Steel, Alloy Steel, Wrought Iron, Cast Iron
- Stainless Steel (active)
- Ni-resist
- Soft Solders, Lead, Tin
- Brasses, Copper, Bronze, Copper-Nickel Alloy
- Nickel, Inconel
- Stainless Steel (passive), Hastelloy
- Titanium
- Silver
- Graphite, Gold, Platinum, Cathodic Protected End
Metals near the top of this list act as anodes and suffer corrosion when coupled with ones nearer the bottom. Those close together corrode more slowly. Coupled metals within one of the groups corrode the least.
HIGH-TEMPERATURE CORROSION
To predict the effect of high-temperature oxidation requires data on: 1) metal composition, 2) atmosphere composition, 3) temperature, and 4) exposure times. It’s not easy to tell what will happen in one case from the results of another. But what is known is that most light metals (those lighter than their oxides) form a non-protective oxide layer that gets thicker as time goes on. The layer forms, spalls and reforms. Other forms of high-temperature corrosion include sulfidation, carburization and nitruration.
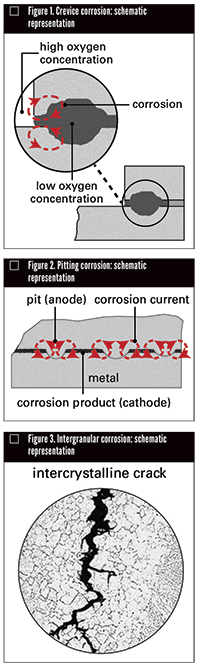
CREVICE CORROSION
This condition can be recognized by its presence in crevices. It sets up differences in solution concentration. The crevice in Figure 1, for example, hinders diffusion of oxygen. The results are that high and low oxygen areas that are anodic cause concentration cells. Metal-ion concentration cells, much like their oxygen counterparts, also strive to balance out concentration differences. Thus, when the solution over a metal contains more metal ions at one point than another, the metal goes into solution where ion concentration is low. Crevice corrosion can be minimized by avoiding: accumulation of deposits on metal surfaces, sharp corners, gaskets joints or other conditions favoring stagnate areas of solution.
UNIFORM ATTACK
This can be seen in a general wasting away of the surface. The condition is found all too often where metals are in contact with acids and other solutions. The corrosion product may form a protective layer on the metal, slowing down corrosion. Or, as in the case of direct chemical attack, the corroded material easily dissolves in the corrosive materials. The problem can be solved by selecting a more corrosion-resistant metal.
PITTING CORROSION
When protective films or layers of corrosion product break down, localized corrosion or pitting occurs (Figure 2). An anode forms where the film breaks, while the unbroken film or corrosion product acts as the cathode. In effect, a closed circuit has been set up.
Some stainless steels in the presence of chloride are susceptible to pitting attack. The breakdown occurs because of some inhomogenity in the metal surface or rough spots.
INTERGRANULAR CORROSION
Intergranular corrosion occurs for a variety of reasons. The result is almost the same—selective attack along the metal’s grain boundaries, destruction of mechanical properties, intercrystalline cracking as shown in Figure 3. Austenitic stainless steels are subject to intergranular corrosion by many corrosives if not properly heat-treated or exposed to sensitizing temperatures of 800–1500°F (427–816°C). This condition can be eliminated by preannealing and quenching from 2000°F (1093°C), by using low carbon stainless steels (C-0.03 max) or stabilized types with columbium or titanium.
TRIBO CORROSION
Similar in method of attack and net effects are erosion corrosion, impingement corrosion and cavitation corrosion. Here’s how they do their damage:
Physical forces from wear break through the protective corrosion scale, dissolving the metal. The effect depends mainly on force and speed. Excessive vibration or flexing of the metal can also have similar effects. Cavitation, a common form of corrosion in pumps and sometimes in valves, depends on the hammer-like effect produced by collapsing air bubbles. Bubbles break down when they pass through a pressure drop area.
STRESS CORROSION CRACKING
Teaming up high-tensile stresses with a corrosive atmosphere is bound to cause trouble. Here’s how it develops:
Tensile stresses build up at metal surfaces under static loading. Corrosive action concentrates stresses and causes them to exceed the metal’s yield point. The result shows up as a local failure. Under continued exposure, the metal alternately corrodes and builds up high-stress concentrations. Eventually, the part may fail. Avoiding this failure can be achieved by early stress relief annealing, proper selections of alloys and design.
CORROSION FATIGUE
In much the same way that static stresses link up with corrosion to produce stress-corrosion cracking, cyclic loads work hand in hand with corrosion to cause corrosion fatigue. Metal failure takes place substantially below the fatigue limit for a non-corrosive condition.
Surprisingly enough, the combined deteriorating effect of these two bed brothers—corrosion and fatigue—is greater than the sum of their individual damages. That’s why it pays to apply the best possible corrosion protection when dealing with metals under alternating stresses.
RELATED CONTENT
-
Cobalt-based Alloy 6 Materials and Boiler Feedwater Service
Q: I’ve heard that cobalt-base Alloy 6 materials should not be used in boiler feedwater service.
-
Do Current Headwinds Affect the Repair or Replace Decision for Valves?
Perspectives from a valve repair company and and OEM on what drives end users to make this determination.
-
The Evolution of the All-Encompassing Ball Valve
The compact design, simplicity of use, ease of repair and wide performance capability have helped to make the ball valve a dominant design in modern industrial applications.










 Unloading large gate valve.jpg;maxWidth=214)